Page 46 - ISAKOS 2021 Newsletter Volume 1
P. 46
CURRENT CONCEPTS
Interpreting Registry Data and Its Effect on a Surgeon’s Decision to Change Implants
Should the Comparator be the Average or the Best?
When surgeons currently examine their own revision risk, or the revision risk of a particular prosthesis or technique, they typically compare it with the average revision risk for all prostheses in the registry. The main disadvantage of this average-comparator approach is that it enables surgeons to maintain a preference for prostheses and techniques that have a higher risk of revision in comparison with other options that have clinically similar outcomes but a lower revision risk. A surgeon with a preference for an option with a higher revision risk may inadvertently form the opinion that their preferred implant choice is acceptable as it is being compared with the mean for all implants rather than to the mean for the lowest-risk implants.
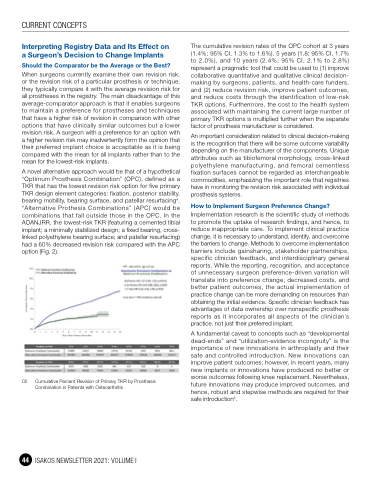
A novel alternative approach would be that of a hypothetical “Optimum Prosthesis Combination” (OPC), defined as a TKR that has the lowest revision risk option for five primary TKR design element categories: fixation, posterior stability, bearing mobility, bearing surface, and patellar resurfacing4. “Alternative Prothesis Combinations” (APC) would be combinations that fall outside those in the OPC. In the AOANJRR, the lowest-risk TKR (featuring a cemented tibial implant; a minimally stabilized design; a fixed bearing, cross- linked polyethylene bearing surface; and patellar resurfacing) had a 60% decreased revision risk compared with the APC option (Fig. 2).
02 Cumulative Percent Revision of Primary TKR by Prosthesis Combination in Patients with Osteoarthritis
The cumulative revision rates of the OPC cohort at 3 years (1.4%; 95% CI, 1.3% to 1.6%), 5 years (1.8; 95% CI, 1.7% to 2.0%), and 10 years (2.4%; 95% CI, 2.1% to 2.8%) represent a pragmatic tool that could be used to (1) improve collaborative quantitative and qualitative clinical decision- making by surgeons, patients, and health-care funders, and (2) reduce revision risk, improve patient outcomes, and reduce costs through the identification of low-risk TKR options. Furthermore, the cost to the health system associated with maintaining the current large number of primary TKR options is multiplied further when the separate factor of prosthesis manufacturer is considered.
An important consideration related to clinical decision-making is the recognition that there will be some outcome variability depending on the manufacturer of the components. Unique attributes such as tibiofemoral morphology, cross-linked polyethylene manufacturing, and femoral cementless fixation surfaces cannot be regarded as interchangeable commodities, emphasizing the important role that registries have in monitoring the revision risk associated with individual prosthesis systems.
How to Implement Surgeon Preference Change?
Implementation research is the scientific study of methods to promote the uptake of research findings, and hence, to reduce inappropriate care. To implement clinical practice change, it is necessary to understand, identify, and overcome the barriers to change. Methods to overcome implementation barriers include gainsharing, stakeholder partnerships, specific clinician feedback, and interdisciplinary general reports. While the reporting, recognition, and acceptance of unnecessary surgeon preference-driven variation will translate into preference change, decreased costs, and better patient outcomes, the actual implementation of practice change can be more demanding on resources than obtaining the initial evidence. Specific clinician feedback has advantages of data ownership over nonspecific prosthesis reports as it incorporates all aspects of the clinician’s practice, not just their preferred implant.
A fundamental caveat to concepts such as “developmental dead-ends” and “utilization-evidence incongruity” is the importance of new innovations in arthroplasty and their safe and controlled introduction. New innovations can improve patient outcomes; however, in recent years, many new implants or innovations have produced no better or worse outcomes following knee replacement. Nevertheless, future innovations may produce improved outcomes, and hence, robust and stepwise methods are required for their safe introduction5.
44 ISAKOS NEWSLETTER 2021: VOLUME I